The Food and Drug Administration approved a record-breaking 45 novel new drug entities and 11 new biologics in 2015. The 45 new drugs include traditional chemical drugs as well as monoclonal and immune-based discoveries.
The latter have created much hope and promise for cures as well as treatment, especially for certain types of cancer.
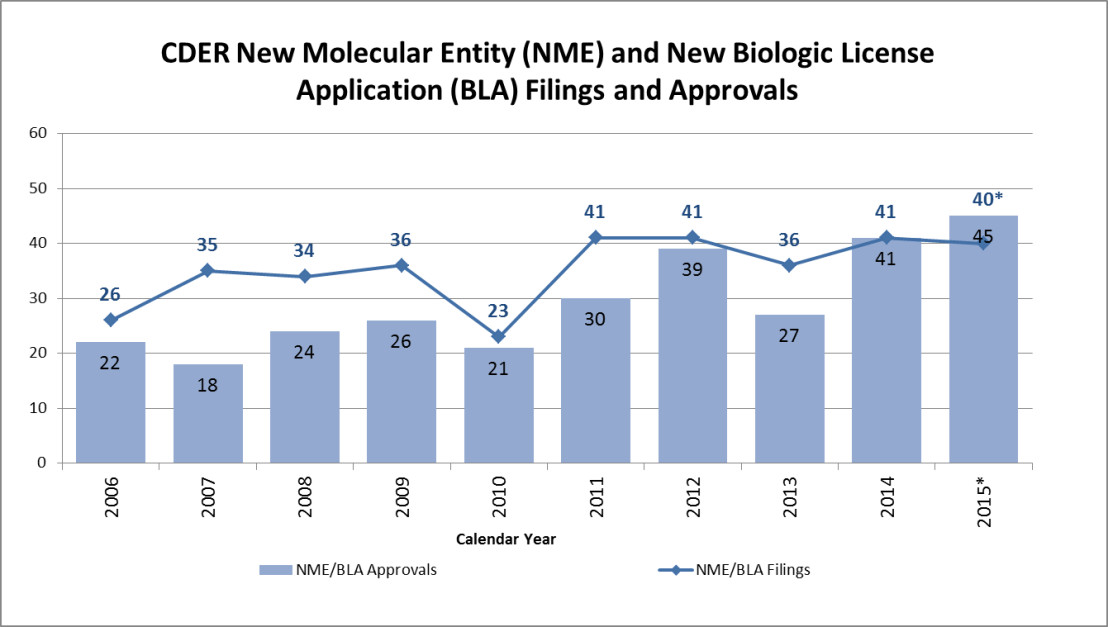
From 2006 until now, the FDA has averaged 28 novel new drug approvals per year. 2015 marked a significant increase in approvals (See chart). Interestingly, 16 of the new approvals were First-in-Class, indicating exciting breakthroughs in categories never before developed. Among these are four drugs for cancer treatment (Darzalex, Empliciti, Ibrance, Unituxin) and four for cardiovascular disease (Corlanor, Entresto, Kanuma, Praluent). Others include Addyi, which treats endocrine disorders, Cosentyx, a treatment for plaque psoriasis, and Nucala, a supplemental treatment for people with severe asthma.
There were also 10 designated breakthrough drugs with clinical trial evidence demonstrating that the drug may result in substantial improvement over other available therapies. Among these are Alecensa for ALK-positive, metastatic non-small cell lung cancer, Darzalex and Empliciti for multiple myeloma, and Ibrance for ER-Positive, HER2-negative breast cancer.
With these additions to current therapies, research continues to make available valuable treatments. It is important to note that, although these agents can offer significant hope to patients, they can also have side effects that will only become evident with more widespread use. Therefore patients and the medical community should be vigilant for adverse events when prescribed these agents. For more detailed information on these new drugs you can visit the FDA Centerwatch website.
*The 2015 filed numbers include those filed in CY 2015 plus those currently pending filing (i.e., within their 60 day filing period) in CY 2015.
– Receipts that received a “Refuse to File” (RTF) or “Withdrawn before filing” (WF) identifier are excluded.
– Multiple submissions (multiple or split originals) pertaining to a single new molecular/biologic entity are only counted once.
– The filed number is not indicative of workload in the Prescription Drug User Fee Act (PDUFA) V Program.
Source: http://www.fda.gov/Drugs/DevelopmentApprovalProcess/DrugInnovation/ucm474696.htm
SUBSCRIBE